Enhancing Disinfection Efforts Using DryDecon™ Technology
Table of Contents
Protecting patients and their healthcare professionals from healthcare-associated infections (HAIs)
In hospitals and related healthcare facilities across the United States, infections acquired by patients during treatment have recently been identified as a significant cause of avoidable illness and death. Research shows that upwards of 5% of patients will experience a HAI after undergoing surgery[1], and approximately 3% of all patients have some form of HAI at any given time[2]. These infections lead to tens of thousands of deaths and have direct medical costs of $28.4 billion to $45 billion each year[3].
A common contributing factor to the increasing rate of HAIs is a reliance on less-effective disinfection strategies within healthcare environments. Current infection control methodologies often fail to reach and remove sufficient percentages of bacteria, fungi, viruses, and other pathogens required to reliably protect patients. Existing procedures also leave behind excess moisture that must be manually wiped away, disperse disinfectants unevenly, and fail to remove microbes hidden deep within microscopic surface cracks. In order to safeguard the well-being and livelihoods of both staff and patients, healthcare facilities must be equipped with the resources to properly neutralize pathogens that cause HAIs.
In an effort to assist the healthcare industry in its mission to protect and support health and wellness, Appliedinfo Partners, Inc. has collaborated with its subsidiary company – hereafter referred to as DryDecon™ Defense – to produce a series of modern disinfection systems. Using our innovative patented DryDecon™ Technology, our systems convert virtually any certified disinfectant solution into a sub-micron aerosol vapor that evenly fills any contained area. Unlike most disinfection methods, the DryDecon™ approach generates particles small enough to reach pathogens within microscopic crevasses and porous materials. Disinfection using DryDecon™ Technology also leaves areas practically dry, eliminating post-treatment wipe-down times. It is our goal to leverage DryDecon™ Technology to support the healthcare industry’s capability to protect its patients in an effective, timely, and thorough manner.
Contributing Factors to HAIs
In order to develop a more effective disinfection strategy, the cause of increased HAIs within healthcare facilities must first be identified. By isolating and inspecting the contributing factors, the shortcomings of existing disinfection processes become clearer. The health and safety of those seeking medical assistance can only be assured if we identify reliable and verifiable solutions to these concerns. Our team has identified three primary factors that must be addressed in order to reduce the rate of HAIs:
Unreachable Threats
Most currently deployed disinfection efforts rely on direct human application, such as spray-and-wipe or mechanical scrubbing efforts. Unfortunately, this means that out-of-reach locations (i.e.; ceilings, light fixtures, elevated shelving, etc.) and overlooked areas (i.e.; air ducts, behind machinery, surface undersides, etc.) regularly do not receive sufficient application of disinfectant to remove harmful pathogens. Even easily reachable, non-porous surfaces contain countless microscopic crevasses that cannot be seen with the naked eye. Trace amounts of viruses, bacteria, and mold spores can collect within these crevasses, where they remain inaccessible to large disinfectant particles.

Antibiotic Resistance
Maintaining a rigorous and thorough disinfection strategy is equally critical to mitigating the spread of infections from pathogens which have grown resistant to antibiotics. As bacteria evolve to resist the effects of commonly prescribed medications, they cause difficult-to-treat, often fatal, infections that spread swiftly between patients. Without exercising proper disinfection methodologies, healthcare facilities can experience sudden outbreaks that can be hard to contain or effectively treat.

Nosocomial COVID-19 Transmission
Beyond typical bacterial and viral infections, healthcare facilities are still struggling to effectively prevent the spread of COVID-19 between patients. While hospitals have attempted to diminish transmission of the virus by housing infected and non-infected patients in separate wings, significant traces of COVID-19 could still be detected within the non-infected patients’ wing[4]. Additionally, current COVID-19 disinfection procedures are time intensive, often taking well over an hour[5], leaving otherwise empty rooms or beds inaccessible to waiting patients. To mitigate the transmission of COVID-19 between patients within a healthcare facility, a disinfection strategy that quickly and reliably eliminates viruses within the air, on surfaces, and within fabrics must be deployed.
Left unchecked, any of these prevailing concerns could cause a catastrophic outbreak that would drastically impact patient safety. Without the ability to rapidly eradicate a significant percentage of harmful pathogens within a room, healthcare professionals must make a choice between exposing patients to a potentially hazardous environment or completely losing access to the affected location. Presently, the only option available involves evacuating affected areas until lengthy “terminal cleaning” procedures can be completed. The Terminal Cleaning process is extremely detailed and time consuming, requiring a significant amount of training that many professionals either never receive or fail to maintain. The events which unfolded at the New Jersey Veteran Homes in Menlo Park and Paramus are proof of this claim.
From 2021 to 2022, the United States Department of Justice (DOJ) conducted five multiday, onsite visits to two long-term care facilities that provide skilled nursing care to veterans and their families. According to the DOJ’s September 2023 report, the failure of both facilities to implement proper clinical care policy or ensure basic staff competency let the virus spread virtually unchecked. Listed among the primary contributing factors for the rampant spread of infection was a failure to sufficiently disinfect surfaces. The results of their research indicated that the number of resident deaths at Menlo Park and Paramus were “substantially higher than the numbers publicly disclosed, and substantially higher than at other facilities”[6].
The Current State of Disinfection Capabilities
Despite lessons learned and policies enacted throughout the COVID-19 pandemic, the rate of HAIs continues to increase within healthcare facilities across the nation. While it may not be possible to prevent all instances of HAIs, we do have the capability to provide healthcare professionals with the tools needed to instate more effective disinfection protocols. It is for this very purpose that our team has developed DryDecon™ Technology.
While many different disinfection methodologies are already deployed by the healthcare industry, no single approach is without notable fault. Most systems fall short in one of the many critical aspects of proper disinfection, such as even dispersal, complete sanitization, or resource efficiency. As such, deciding which form of disinfection to utilize often involves selecting the most convenient option instead of the option that would provide the most beneficial outcome. Figure 1 provides an overview of the most common forms of disinfection utilized within healthcare facilities today, as well as a brief overview of their notable faults.
| Spray & Wipe | Does not guarantee complete dispersal of disinfectant. Wiping causes cross contamination. |
| Electrostatic Sprayers | Does not sanitize the air. Large particles fail to clean small crevices. Leaves behind moisture. |
| Antimicrobial Coatings | Reduces some contaminant loads, but fails to provide complete protection. |
| 24/7 Air & Surface | Reduces some contaminant loads, but falls short of complete disinfection (i.e., Photocatalytic Oxidation). |
| Air Scrubbers | Costly and only cleans the air that passes through the handler. Does not fully treat the air or surfaces. |
| Conventional Foggers | Fails to deliver disinfectant effectively due to wet fog physics resulting in soaked “hot spots”. |
| Gas | Disinfectant disperses evenly, but requires extensive time and dangerous chemical concentrations. |
While there is no single flaw that reduces the efficacy of all conventional disinfection methodologies, a common contributing factor is that most procedures fail to penetrate deep enough into surfaces to properly destroy all traces of a harmful agent. Microscopic contaminant particles can remain hidden deep within surface cracks or crevices that are too small to be seen. People who later come into contact with surfaces that have not been disinfected using a method which penetrates to this level still risk becoming affected by these hidden particles. A successful multi-faceted disinfection protocol must have the capability to thoroughly destroy contaminants, including concentrations which are too small to see. DryDecon™ Technology was created with this specific requirement in mind.
Nebulizers, humidifiers, and foggers function by converting disinfectant solutions into small droplets that are emitted into the environment as a fog. Yet, these devices utilize an inefficient conversion process that results in the development of large droplet particles. The relatively large average size of these particles causes them to be heavy, which impedes the device’s ability to effectively disperse the disinfectant. As a result, the disinfectant is not spread evenly, does not penetrate into microscopic surface crevices, and cannot reach the underside of many elevated surfaces. Instead, the product concentrates toward the floor and creates excessive moisture in pocket locations.
Figure 2: The Flaws in Current Disinfection Procedures
Enhancing Disinfection Capabilities with DryDecon™ Technology
DryDecon™ Technology addresses the shortcomings of current disinfection strategies by ensuring that only the smallest particles are dispersed from the unit. Instead of a thick and heavy fog, our systems automatically convert disinfectant solutions into a sub-micron aerosol vapor. The dispersed vapor is therefore lighter and diffuses more evenly throughout an enclosed space. As a result, all of the air and surfaces within the room are quickly and thoroughly treated without leaving behind moisture. Furthermore, by only allowing the smallest particles to leave the unit, we ensure that residual pathogens located deep within small crevices and fabrics are reached and completely removed.
Most commonly used fogging devices produce particles averaging from 5-25 microns (µm) in size, which is too large to penetrate microscopic surface cracks . Conversely, DryDecon™ Technology produces particles at a median size of .6 µm, with 46% of the overall released particles being less than 0.5 µm in size, 79% under 1 µm, and 99% under 2.5 µm. The extremely small size of these particles has allowed our team to achieve a deeper degree of disinfection penetration. The lighter particles also fill the air and reach distant surfaces more evenly, resulting in a greater levels of disinfection for all areas within an enclosed space.

Figure 3: Deeper Disinfection with DryDecon™ Technology
Restricting the release of larger particles also decreases the amount of moisture added to the air, thereby lowering condensation levels within treated areas. Reducing condensation is integral to deep layer disinfection, as cohesion between larger particles creates a barrier that prevents smaller particles from entering microscopic voids within a surface. By preventing that barrier from forming, DryDecon™ Technology allows disinfectant to enter these voids and remove hidden pathogens. Additionally, our process eliminates the time associated with removing residual wetness and reduces the risk of damaging equipment that may be sensitive to moisture.
DryDecon™ Technology can be design to fit to a variety of form factors in order to meet the specific needs of any healthcare facility. Based on the size of the space to be disinfected, our team can produce units as small as a common thermos or as large as a trailer. Furthermore, DryDecon™ units can be customized for use with a variety of different power supplies. This allows our team to ensure that there is a DryDecon™ unit compatible with nearly any disinfectant requirement.
Microchem Laboratory – August 2022 (2 Tests)
Microchem Laboratory, a leader in rigorous third-party scientific testing, was employed to confirm how effectively DryDecon™ Technology could deliver disinfectant[7]. Our system was used to perform two separate experiments against three primary agents: Salmonella, Staphylococcus, and Black Mold.
During the first test, Microchem scientists identified what Log kill each disinfectant agent could achieve when delivered by a DryDecon™ unit. Their process involved simulating a conveyor vapor tunnel or laundry cart wash type application. The researchers removed the contaminant from the sub-micron aerosol vapor after 10 minutes and subjected it to an agent which neutralizes the disinfectant. From there, if any contaminant had remained, it was free to flourish over a three-day incubation period. Results proved from 99.99% to 99.999% elimination showing the DryDecon™ system was able to deliver the disinfectant product and achieve the product’s expected kill rates proving DryDecon™ to be an effective delivery system.
The second test involved discovering DryDecon™ Technology’s capability to disinfect without follow-up rinsing or wiping activity. This test would determine how much contaminant could be killed in an enclosed space when the disinfectant is not neutralized or directly interacted with in any way. The DryDecon™ system dispersed sub-micron aerosol vapor into a room for 10 minutes and, after a three-day incubation period, absolutely no new growth had occurred. This suggested a residual elimination quality of the DryDecon™ process and provides expectations of performance in a real-world scenario.
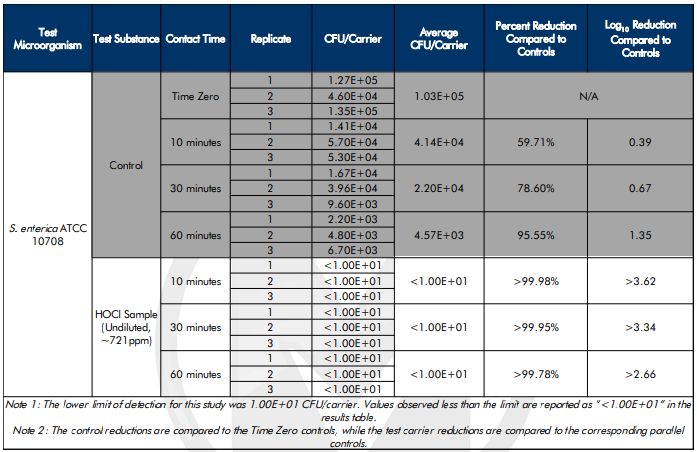
Figure 4: Sample of Microchem’s DryDecon™ Experiment Results
Fordham University – February 2023
Dr. Martin Sanzari, Ph.D., Director of Engineering Physics at Fordham University, tested DryDecon™ Technology’s ability to produce smaller water particles on average when compared to commonly used disinfection units[8]. Dr. Sanzari was able to prove that our DryDecon™ systems generate 2.5x less humidity in the surrounding environment. His tests showed that by producing less humidity, our units cause fewer large water particles to form.
During testing, Dr. Sanzari was able to demonstrate that our virtually invisible sub-micron particles were able to populate the test room with over 8,000 particles per cmᶟ that were less than 1µm in size after only two minutes of run time. Even after 30,000 total particles per cmᶟ existed, only 150 were larger than 4µm and over 99% were less than 2.5µm. He also found that the particles were distributed rapidly and were barely visible throughout the test. These results support the claim that DryDecon™ Technology achieves a deeper level of disinfection with reduced clean-up time when compared to currently deployed systems.
Number of Particles per cmᶟ at Various Sizes
| DryDecon™ | < 0.5 µm | % | .5 – 1 µm | % | 1 – 2.5 µm | % | 2.5 – 4 µm | % | 4 – 10 µm | % | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Machine Powered | 17 | 88% | 2 | 11% | 0 | 0% | 0 | 0% | 0 | 0% | 20.29 |
| Fog Began | 16 | 84% | 2 | 13% | 0 | 2% | 0 | 0% | 0 | 0% | 19.61 |
| 1 min | 2,552 | 64% | 926 | 23% | 399 | 10% | 78 | 2% | 11 | 0% | 3,966.91 |
| 2 min | 5,468 | 52% | 3,083 | 29% | 1,575 | 15% | 308 | 3% | 44 | 0% | 10,478.91 |
| 3 min | 9,577 | 50% | 5,866 | 31% | 3,064 | 16% | 600 | 3% | 86 | 0% | 19,193.18 |
| 4 min | 11,653 | 48% | 7,699 | 32% | 4,096 | 17% | 802 | 3% | 114 | 0% | 24,365.35 |
| 5 min | 14,003 | 46% | 9,947 | 33% | 5,376 | 18% | 1,053 | 3% | 150 | 0% | 30,530.28 |
The Environmental Protection Agency (EPA) Approves Air Kill Claim Capabilities (October, 2022)
Beyond the scientific verification of our technology’s capabilities provided above, the U.S. Government has recently provided official approval of air kill claim capabilities[9]. In October 2022, the EPA registered the first ever product for use against airborne bacteria and viruses in residential and commercial areas. This is a landmark event, as it provides a government-approved protocol under which air kill tests can be performed. Moving forward, this will support our assertion that DryDecon™ Technology verifiably disinfects the entirety of an enclosed space, including the air.
DryDecon™ Technology Disinfects and Deodorizes Rental Home of Cat Urine (April, 2023)
Rytech Restoration, a 30 years old, national property damage mitigation business, received a request to disinfect a two-story rental home located in St. Augustine Beach, Florida. The client explained that the last person who rented the house had abandoned the property and left two cats to roam freely throughout the 1,500-square-foot home. Locked within the house, the cats began to urinate on all of the floors and surfaces throughout the building. Two weeks later, the owner entered the house to discover that the noxious odor of cat urine could be detected in every room within the building.
Rytech technicians inspected the property and discovered that the level of contamination was extensive. The degree of saturation was so intense that the technicians needed to remove the building’s carpets and pry away floorboards to access the source of the scent. Their team cleaned every surface and wall within the property, including the plywood subflooring, using hypochlorous acid (HOCl) and traditional scrubbing methods. The lingering odor still prevailed several days later, as the urine had soaked so deeply into the crevices of the subflooring that it couldn’t be removed using common cleaning protocols.
Using DryDecon™ Technology, the Rytech technicians were able to fully disinfect and deodorize the rental home – even down to the cellular structure of the subflooring – in less than two hours. Not only was the intense cat urine odor completely removed, but it also never returned. In response to this outstanding performance, leadership at Rytech Restoration wrote a letter of recommendation that detailed the benefits of using DryDecon™ Technology. The letter further highlighted the praise they received from their client, which they attributed to the quality of work made possible thanks to our technology.
Potential Healthcare Use Cases
As stated previously, healthcare facilities must implement a truly effective and efficient disinfection strategy in order to mitigate the risk of widespread transmission of harmful pathogens. Our team’s DryDecon™ Technology provides healthcare professionals with a means to more rapidly and thoroughly
disinfect a variety of enclosed spaces. Our approach offers a base technology that addresses bacteria, viruses, and fungi using a scalable, configurable delivery system to support a wide range of use cases. Not only do our systems support deeper levels of disinfection, but they also facilitate faster room turnover times.
We specifically designed DryDecon™ Technology to establish and maintain a higher level of safety and hygiene across a long list of indoor environments. From nursing home residential facilities to hospital waiting rooms, our disinfection systems reach and remove nearly all residual pathogens from within an enclosed space without the threat of causing damage to equipment or electronics. Our technology is also proven safe for utilization in areas with strict disinfection requirements, such as operating rooms and pharmaceutical warehouses. DryDecon™ Technology prevents these critical locations from shutting down or ceasing operations for extensive periods during the disinfection process.
Standard size DryDecon™ units are highly mobile, and come complete with a sturdy handle and wheels. This form factor makes it easy for a single operator to quickly push or pull the machine between rooms. Its portability also allows healthcare professionals to quickly deploy a DryDecon™ unit into a room that may need to undergo immediate emergency disinfection procedures. By offering a capability to undergo rapid disinfection at a moment’s notice, DryDecon™ Technology provides healthcare professionals with the tools and process required to address pathogens both on a scheduled basis and at the moment of need.
When designing DryDecon™ Technology, our aim was to produce a modular solution. As such, we focused on developing a device that could support the elimination of multiple viruses, bacteria, fungi, and other potential contaminants. We achieved this goal by constructing a system that can efficiently disperse virtually any certified disinfectant solution, thereby extending the list of harmful pathogens that could be targeted. Presently, DryDecon™ Technology has the ability to serve as the primary disinfecting strategy against COVID-19, Avian Influenza, Clostridium Difficile (C.Dif.), Salmonella, Staphylococcus (Staph), Escherichia coli (E. Coli), as well as various other threats.
Conclusion
On a daily basis, our country’s healthcare professionals strive to safeguard the safety and well-being of those suffering from illness, injury, and the maladies of old age. A significant portion of their mission involves providing a thoroughly safe and sanitary environment for patients to receive treatment and recover. Far too often, however, healthcare facilities do not have the time, tools, or training to employ a disinfection strategy that reliably eliminates harmful pathogens beyond the surface level. Our team has developed DryDecon™ Technology with the specific goal of addressing these concerns and facilitating better disinfection capabilities for the healthcare industry.
| Even distribution of virtually any certified disinfectant solution |
| Removes contaminants in the air, on all surfaces, and within microscopic crevices |
| Leaves treated areas dry |
| Efficient use of disinfectant |
| Effective at achieving below threshold level of clean |
| Lower environmental impact |
| Scalable to support any requirement |
| Safe concentrations of disinfectant are effective |
| Requires minimal maintenance & user training |
Our DryDecon™ systems have been proven to be superior to traditional disinfection strategies in performance, cost, upkeep, ease of operation, versatility, scalability, and durability. Instead of relying on traditional methodologies which fail to penetrate deep into surfaces and fully remove contaminants, we offer a verified approach to complete disinfection that is portable and doesn’t leave behind a layer of moisture. By relying on DryDecon™ Technology, the healthcare industry will gain the ability to disinfect facilities and equipment at a sub-micron level, thereby instating a more efficient method of removing lingering pathogens and establishing safer environments for both staff and patients.
References
[1] Heather L Evans, MD, MS, Traci L Hedrick, MD, MS, FACS, FACRS. “Overview of the evaluation and management of surgical site infection”. (Apr 01, 2022). https://www.uptodate.com/contents/overview-of-the-evaluation-and-management-of-surgical-site-infection
[2] Centers for Disease Control and Prevention. “HAI and Antibiotic Use Prevalence Survey”. (February 25, 2022). https://www.cdc.gov/hai/data/index.html
[3] Stone PW. Economic burden of healthcare-associated infections: an American perspective. Expert review of pharmacoeconomics & outcomes research. 2009;9(5):417–22. doi: 10.1586/erp.09.53 PMC2827870.
[4] Grimalt JO, Vílchez H, Fraile-Ribot PA, Marco E, Campins A, Orfila J, van Drooge BL, Fanjul F. Spread of SARS-CoV-2 in hospital areas. Environ Res. 2022 Mar;204(Pt B):112074. doi: 10.1016/j.envres.2021.112074. Epub 2021 Sep 20. PMID: 34547251; PMCID: PMC8450143.
[5] National Union of Healthcare Workers. “Safety for EVS Workers during the COVID-19 Pandemic.” (2023): https://nuhw.org/wp-content/uploads/EVS-COVID-leaflet-1-page-20200416FINAL.pdf.
[6] United States Department of Justice – Civil Rights Division. “Investigation of the New Jersey Veterans Memorial Homes at Menlo Park and Paramus.” (2023): https://www.justice.gov/d9/2023-09/njveteranshomesfindings.report.pdf.
[7] Study Identification #: NG20073 – A1 (August 4, 2022 – August 11, 2022), Study Identification #: NG20231 (September 13, 2022 – September 30, 2022)
[8] Dr. Marty Sanzari. Particle Count, Relative Humidity, Specific Humidity and Dew Point Temperature Measurements Conducted Using the DryDeCon™ Technology (February 24, 2023)
[9] “EPA Registers Air Sanitizer for Residential and Commercial Use Against Influenza and Coronavirus”, https://www.epa.gov/pesticides/epa-registers-air-sanitizer-residential-and-commercial-use-against-influenza-and